CLEVIPREX® (clevidipine)
Health Economics and Value Team
Discover the potential clinical and economic outcomes of using CLEVIPREX
The CLEVIPREX Value Model
Choosing IV antihypertensives depends on clinical context and patient factors. The CLEVIPREX Value Model uses ISPOR methodology to provide valuable information to a payor, formulary committee, or other similar entity with knowledge and expertise in the area of health economics analysis, carrying out responsibilities for the selection of drugs for coverage or reimbursement.
CLEVIPREX Value Model goals
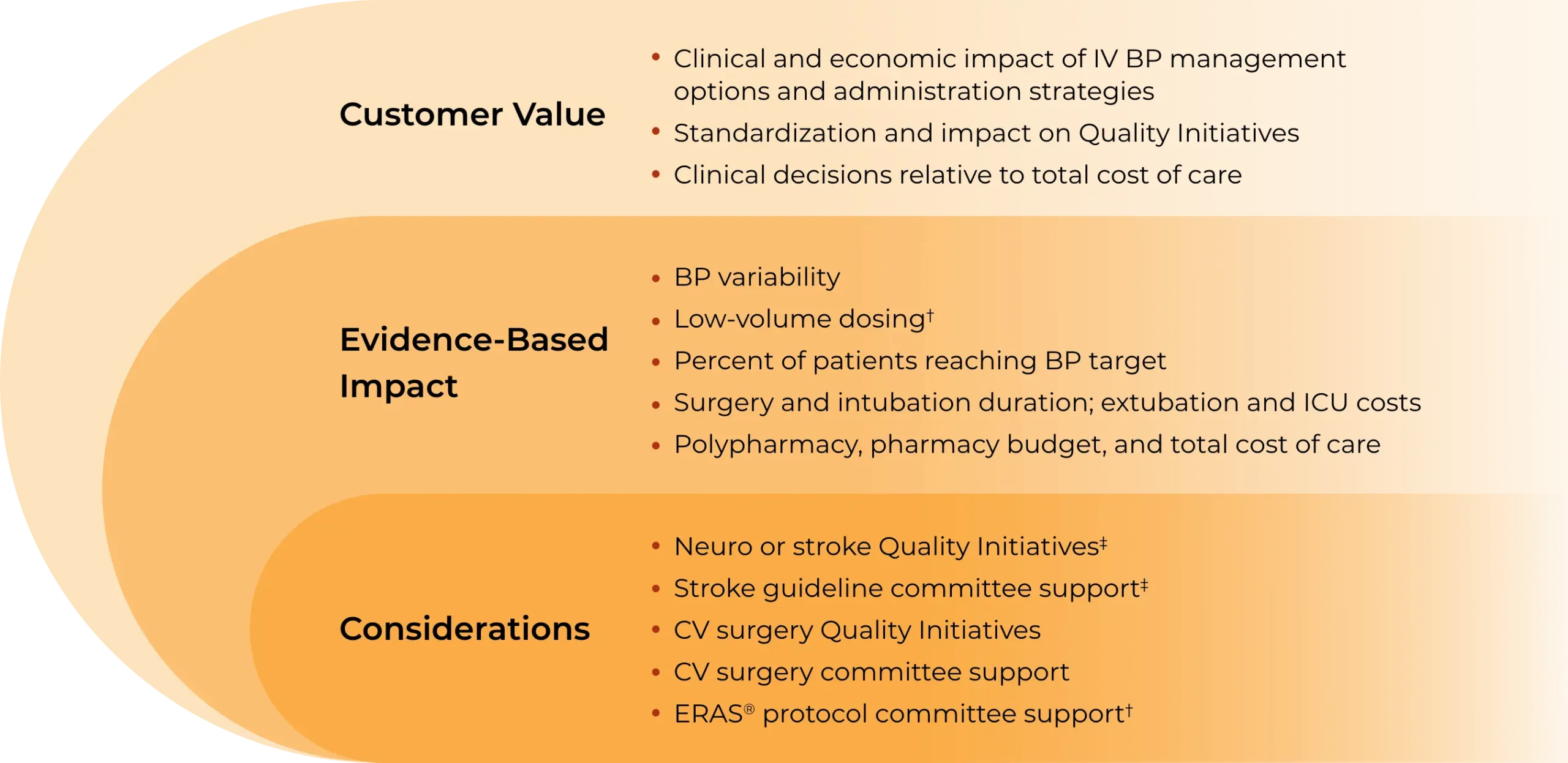
Foster an understanding of IV antihypertensive options and patient outcomes, dosing, adverse event rates, and potential cost implications
Quantify costs and health impact from the unique perspective of US institutions, including hospitals, cardiac suites, emergency departments, and integrated delivery networks
Estimate the clinical outcomes and economic impact of using CLEVIPREX over a 1- to 3-year time horizon in relevant patient populations
CLEVIPREX Value Model inputs and outputs*
The CLEVIPREX Value Model is considered health economics and value information. It is important to note that overall economic impact is dependent on specific patient populations, institutional practices, and local cost structures.
BP=blood pressure; CV=cardiovascular; ERAS=Enhanced Recovery After Surgery; ICU=intensive care unit; ISPOR=International Society for Pharmacoeconomics and Outcomes Research; IV=intravenous.
This information can be provided to a payor, formulary committee, or other similar entity with knowledge and expertise in the area of the health economics analysis, carrying out its responsibilities for the selection of drugs for coverage or reimbursement.
Learn more about CLEVIPREX & the Value Model

Connect with a Health Economics and Value Specialist today to learn more about the CLEVIPREX Value Model
Important Safety Information
CLEVIPREX® (clevidipine) Injectable Emulsion is contraindicated in patients with:
- Allergies to soybeans, soy products, eggs, or egg products;
- Defective lipid metabolism seen in conditions such as pathologic hyperlipemia, lipoid nephrosis, or acute pancreatitis if it is accompanied by hyperlipidemia; and
- Severe aortic stenosis.
CLEVIPREX® is intended for intravenous use. Use aseptic technique and discard any unused product within 12 hours of stopper puncture.
Hypotension and reflex tachycardia are potential consequences of rapid upward titration of CLEVIPREX®. If either occurs, decrease the dose of CLEVIPREX®. There is limited experience with short-duration therapy with beta-blockers as a treatment for CLEVIPREX®-induced tachycardia. Beta-blocker use for this purpose is not recommended.
CLEVIPREX® contains approximately 0.2 g of lipid per mL (2.0 kcal). Lipid intake restrictions may be necessary for patients with significant disorders of lipid metabolism.
Dihydropyridine calcium channel blockers can produce negative inotropic effects and exacerbate heart failure. Monitor heart failure patients carefully.
CLEVIPREX® is not a beta-blocker, does not reduce heart rate, and gives no protection against the effects of abrupt beta-blocker withdrawal. Beta-blockers should be withdrawn only after a gradual reduction in dose.
Patients who receive prolonged CLEVIPREX® infusions and are not transitioned to other antihypertensive therapies should be monitored for the possibility of rebound hypertension for at least 8 hours after the infusion is stopped.
There is no information to guide use of CLEVIPREX® in treating hypertension associated with pheochromocytoma.
Most common adverse reactions for CLEVIPREX® (>2%) are headache, nausea, and vomiting.
Indication
CLEVIPREX® (clevidipine) is a dihydropyridine calcium channel blocker indicated for the reduction of blood pressure (BP) when oral therapy is not feasible or not desirable.
Please see Full Prescribing Information.