KENGREAL® (cangrelor)
Health Economics and Value Team
Discover the potential clinical and economic outcomes of using KENGREAL
The KENGREAL Value Model
Using KENGREAL in high-risk and complex PCI cases may have an economic impact at your institution. The KENGREAL Value Model demonstrates how using KENGREAL versus clopidogrel (with or without GPIIb/IIIa) may affect health economics, considering the possible implications of ischemic and bleeding events across a 1- to 3-year horizon.
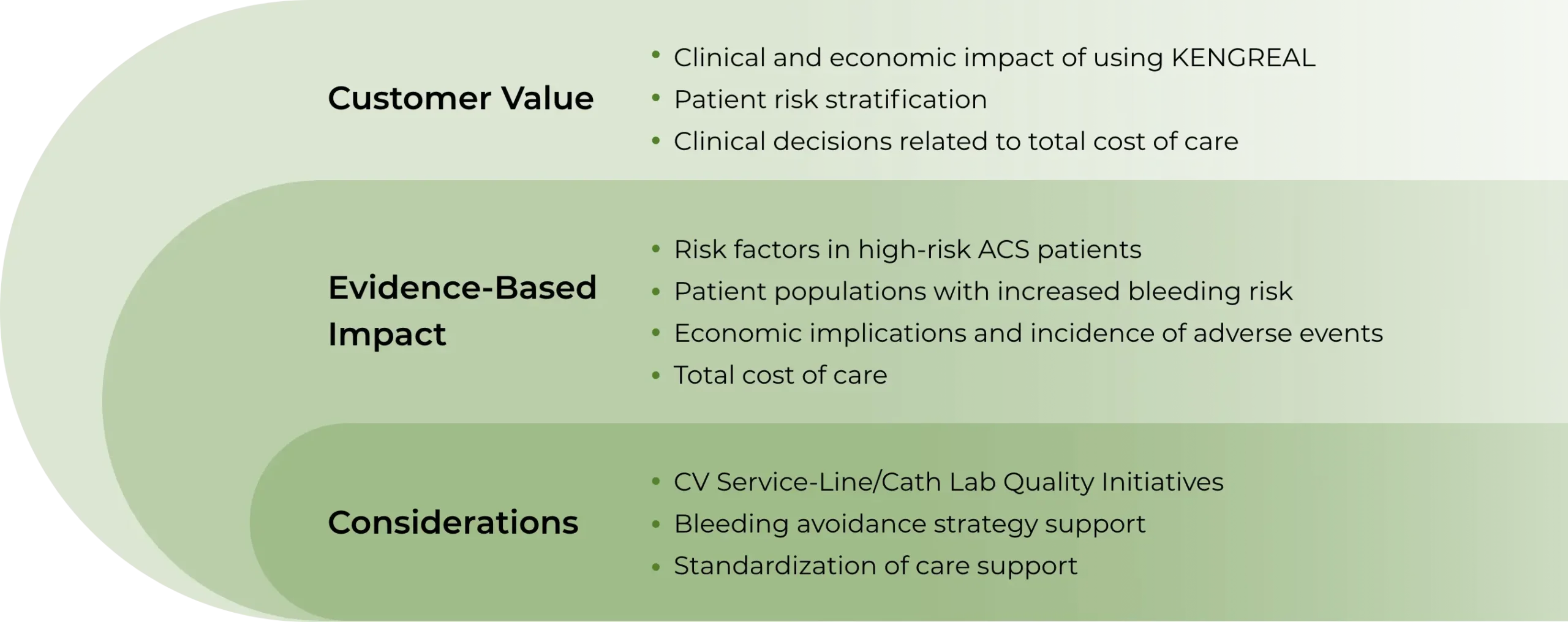
KENGREAL Value Model goals
Establish institution-specific baseline for anti-platelet therapy utilization
Identify the most valuable use criteria for KENGREAL
Demonstrate KENGREAL’s potential impact and budget implications in appropriate patient populations
Support monitoring with 6- to 12-month repeat analysis options
KENGREAL Value Model inputs and outputs
The KENGREAL Value Model is considered health economics and value information. It is important to note that overall economic impact is dependent on specific patient populations, institutional practices, and local cost structures.
ACS=acute coronary syndrome; CV=cardiovascular; GPIIb/IIIa=glycoprotein IIb/IIIa inhibitor; PCI=percutaneous coronary intervention.
This information can be provided to a payor, formulary committee, or other similar entity with knowledge and expertise in the area of the health economics analysis, carrying out its responsibilities for the selection of drugs for coverage or reimbursement.
Learn more about KENGREAL & the Value Model

Connect with a Health Economics and Value Specialist today to learn more about the KENGREAL Value Model
Important Safety Information
KENGREAL® (cangrelor) for Injection is contraindicated in patients with significant active bleeding.
KENGREAL® is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Drugs that inhibit platelet P2Y12 function, including KENGREAL®, increase the risk of bleeding. In CHAMPION PHOENIX, bleeding events of all severities were more common with KENGREAL® than with clopidogrel. Bleeding complications with KENGREAL® were consistent across a variety of clinically important subgroups. Once KENGREAL® is discontinued, there is no antiplatelet effect after an hour.
The most common adverse reaction is bleeding.
Indication
KENGREAL® (cangrelor) for Injection is a P2Y12 platelet inhibitor indicated as an adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
Please see Full Prescribing Information.