CUROSURF® (poractant alfa)
Health Economics and Value Team
Discover the potential clinical and economic outcomes of using CUROSURF
The CUROSURF Value Model
The interactive CUROSURF Value Model customizes inputs for your institution and outlines potential clinical outcomes and cost implications of using standard surfactant administration versus early rescue surfactant administration.
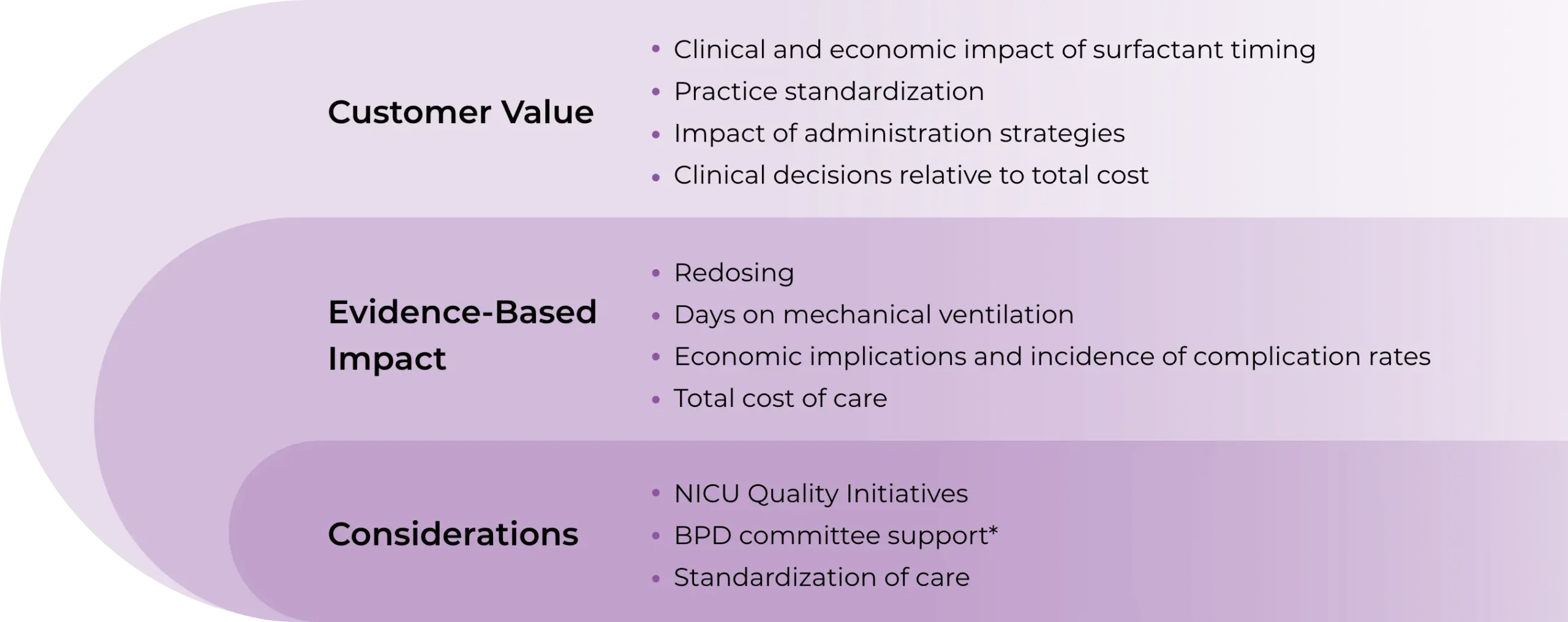
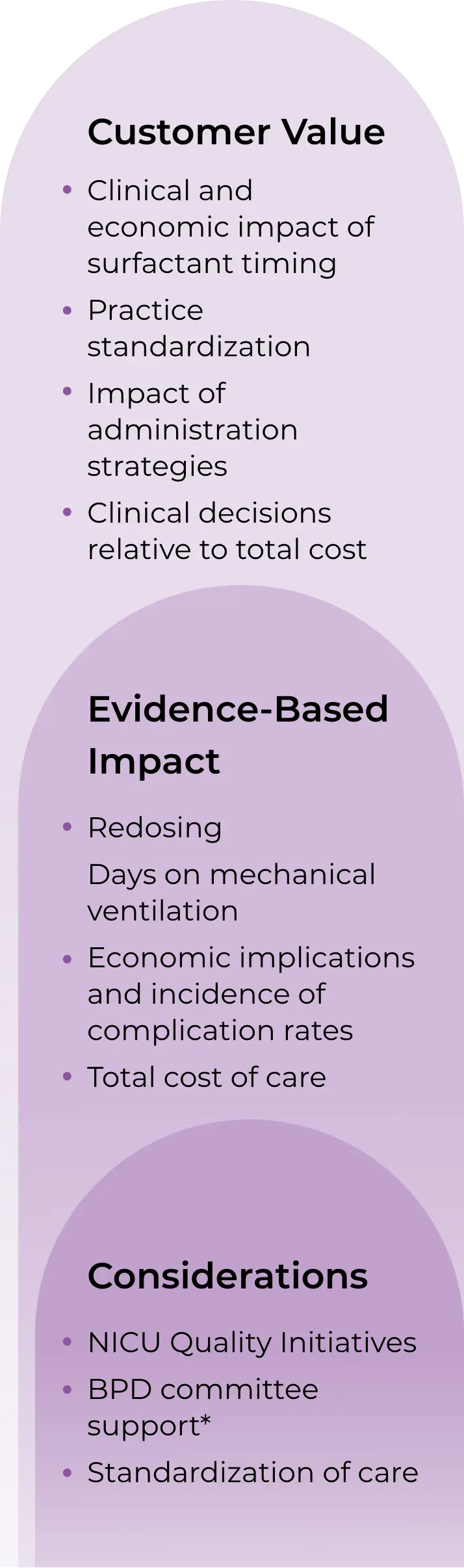
CUROSURF Value Model goals1
Demonstrate potential cost implications of standard surfactant administration versus early rescue surfactant administration as it relates to clinical outcomes
Provide key information for US healthcare decision-makers like quality personnel, pharmacy administration, and P&T Committee members
Support payors or formulary committees in drug selection for coverage or reimbursement
CUROSURF Value Model inputs & outputs
CUROSURF was assumed to be used in all treatment strategies. This model is considered health economics and value information. It is important to note that overall economic impact is dependent on specific patient populations, institutional practices, and local cost structures.
BPD=bronchopulmonary dysplasia; FiO2=fraction of inspired oxygen; NICU=neonatal intensive care unit; P&T=Pharmacy and Therapeutics; RDS=respiratory distress syndrome.
This information can be provided to a payor, formulary committee, or other similar entity with knowledge and expertise in the area of the health economics analysis, carrying out its responsibilities for the selection of drugs for coverage or reimbursement.
Learn more about CUROSURF & the Value Model

Connect with a Health Economics and Value Specialist today to learn more about the CUROSURF Value Model
Important Safety Information
CUROSURF® (poractant alfa) is intended for intratracheal use only. The administration of exogenous surfactants, including CUROSURF, can rapidly affect oxygenation and lung compliance. Therefore, infants receiving CUROSURF should receive frequent clinical and laboratory assessments so that oxygen and ventilatory support can be modified to respond to respiratory changes.
CUROSURF should only be administered by those trained and experienced in the care, resuscitation, and stabilization of preterm infants.
Transient adverse reactions associated with administration of CUROSURF include bradycardia, hypotension, endotracheal tube blockage, and oxygen desaturation. These events require stopping CUROSURF administration and taking appropriate measures to alleviate the condition. After the patient is stable, dosing may proceed with appropriate monitoring.
Pulmonary hemorrhage, a known complication of premature birth and very low birth-weight, has been reported with CUROSURF. The rates of common complications of prematurity observed in a multicenter single-dose study that enrolled infants 700–2000 g birth weight with RDS requiring mechanical ventilation and FiO2 ≥ 0.60 are as follows for CUROSURF 2.5 mL/kg (200 mg/kg) (n=78) and control (n=66; no surfactant) respectively: acquired pneumonia (17% vs. 21%), acquired septicemia (14% vs. 18%), bronchopulmonary dysplasia (18% vs. 22%), intracranial hemorrhage (51% vs. 64%), patent ductus arteriosus (60% vs. 48%), pneumothorax (21% vs. 36%) and pulmonary interstitial emphysema (21% vs. 38%).
Indication
CUROSURF® (poractant alfa) Intratracheal Suspension is indicated for the rescue treatment of Respiratory Distress Syndrome (RDS) in premature infants. CUROSURF reduces mortality and pneumothoraces associated with RDS.
Please see Full Prescribing Information.
Reference: 1. Data on file. Chiesi USA, Inc.